
Gas compressibility factor Z: Ideal gas vs Real gas
4.5 (669) In stock

4.5 (669) In stock
Gas compressibility factor, Z, and Gas compressibility are not the same. Gas compressibility factor Z is the ratio of the gas volume at a given temperature and pressure to the volume the gas would occupy if it were an ideal gas at the same temperature and pressure.

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

Centrifugal Compressor Surging Causes

What is the compressibility factor? What is its value an ideal gas? How does it to understand the extent of deviation of a gas from ideal behavior?

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

Pressure Maintenance in the Groningen Gas Field (Part III)

Non-ideal behavior of gases (article)

Centrifugal Compressor Surging Causes

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange
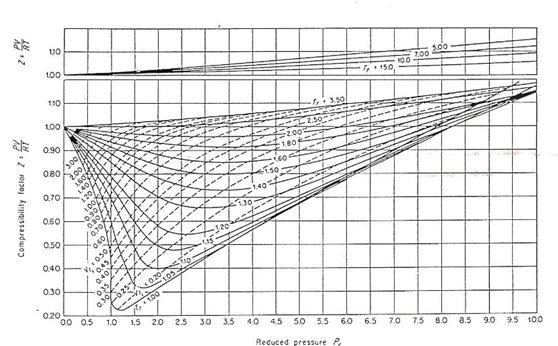
How the ideal gas law helped us creating a software tool called Fluidat

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Acidizing 1. Types of Acid Treatments

Compressibility Factor Charts - Wolfram Demonstrations Project

GOR is not a good indicator of reservoir fluid type in tight liquid-rich shale plays