
Ideal gas law, Definition, Formula, & Facts
4.9 (126) In stock

4.9 (126) In stock
Ideal gas law, relation between the pressure P, volume V, and temperature T of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: PV =

Partial pressure - Wikipedia
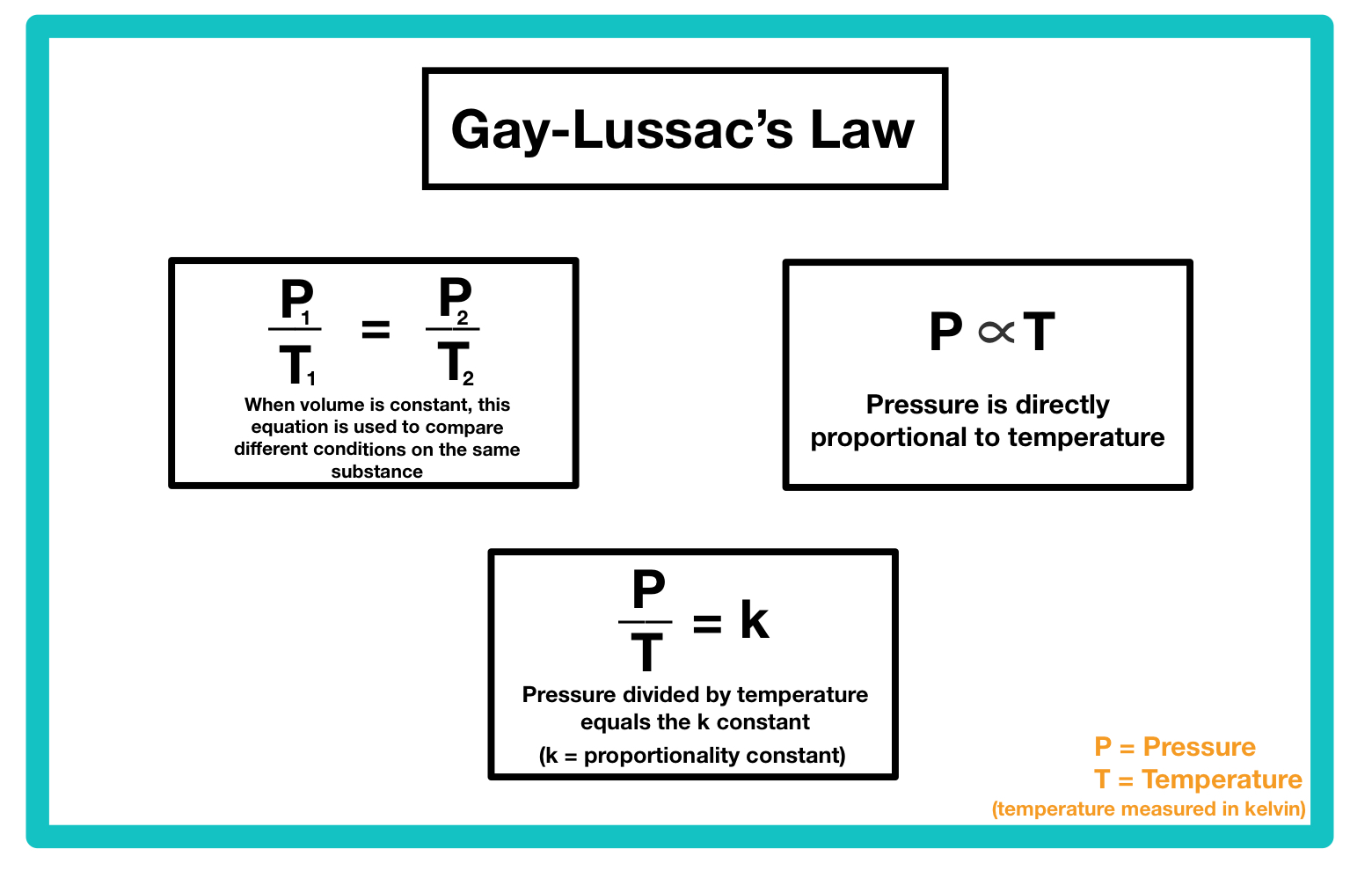
Gay-Lussac's Law of Ideal Gasses Study Guide - Inspirit Learning Inc
:max_bytes(150000):strip_icc()/143058853-56a12f375f9b58b7d0bcdc3c.jpg)
Combined Gas Law Definition and Examples

How to Use the Ideal Gas Law to Calculate a Change in Volume

The ideal gas law (PV = nRT) Intermolecular forces and

Ideal Gas Constant (R) - Universal Gas Constant

The Gas Laws - Statements, Formulae, Solved Problems
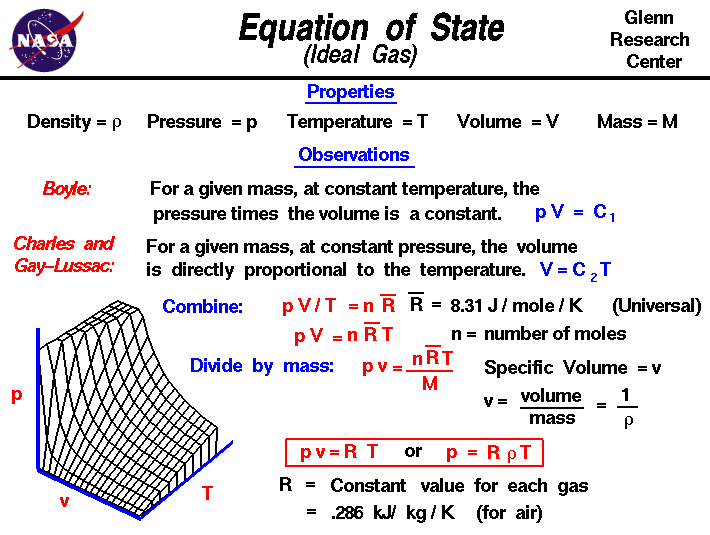
Equation of State

Lesson Explainer: Boyle's Law

Charle's Law - Definition, Formula, Derivation, Application

Charles' Law, Formula, Units & Application - Lesson