
Non-ideal behavior of gases (article)
4.9 (692) In stock

4.9 (692) In stock
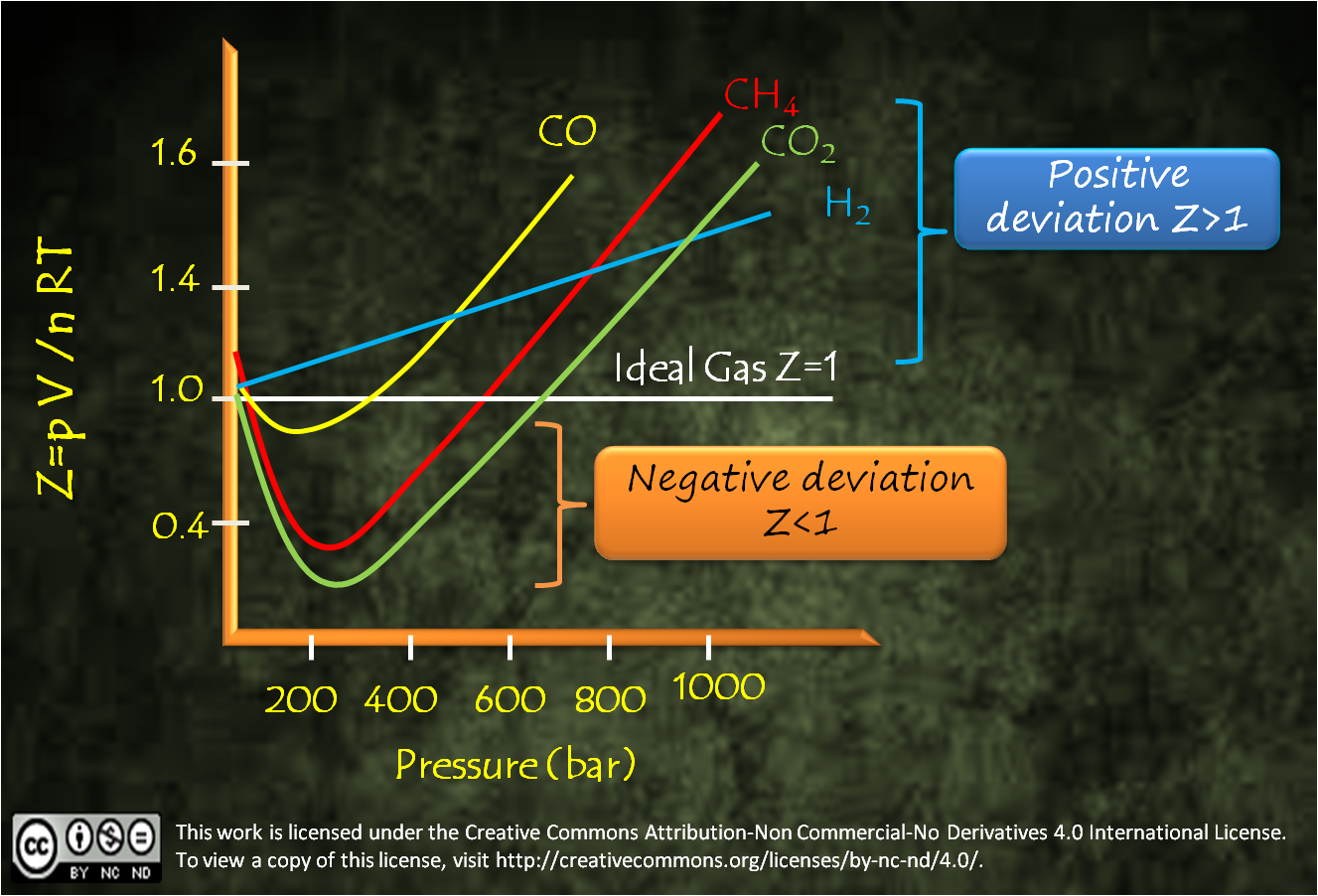
Chemistry!!! Not Mystery : Do Real Gases Behave Ideally?

gas laws - Among hydrogen, helium and carbon dioxide, which gas would behave most like ideal gas and why? - Chemistry Stack Exchange
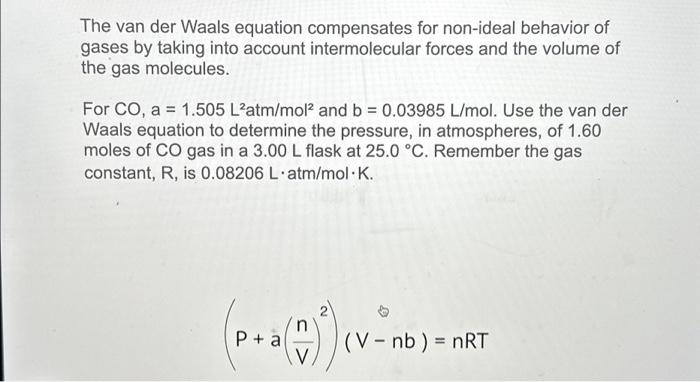
Solved The van der Waals equation compensates for non-ideal

What is an ideal gas? Explain.

OneClass: At high pressures, real gases do not behave ideally. Calculate the pressure exerted by 12.0

PPT - Unit 4 Section A.15-A.16 PowerPoint Presentation, free download - ID:5719053
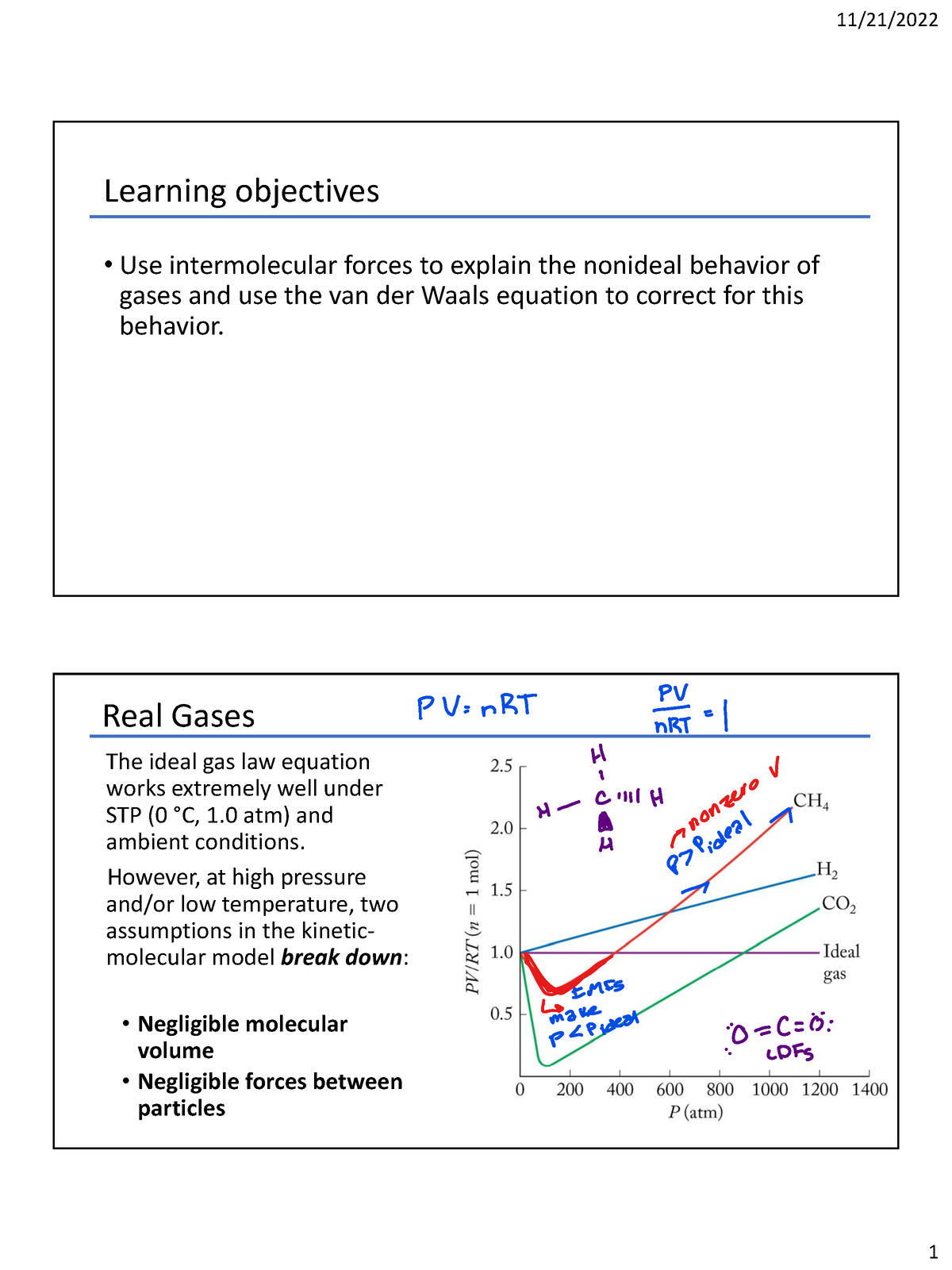
11.28.22 Chapter 9D real gases student - Learning objectives • Use intermolecular forces to explain - Studocu
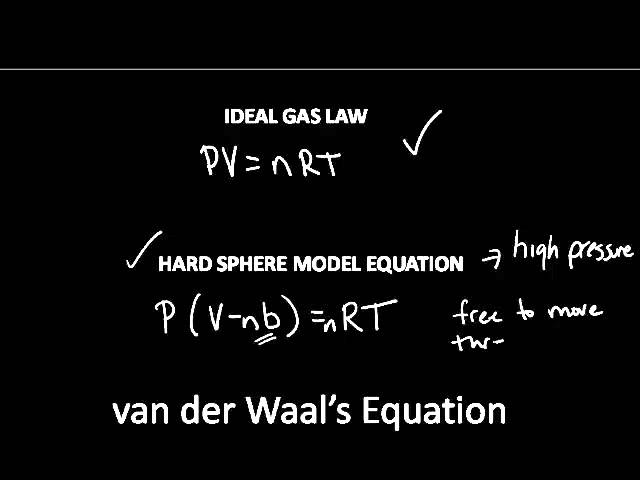
Ideal vs Non Ideal Gas Behavior

Ideal gas - Wikipedia