
Thermodynamic Properties Property Table w Property Table -- from
4.5 (709) In stock

4.5 (709) In stock
Universal Gas Constant Universal gas constant is given on R u = kJ/kmol-K = kPa-m 3 /kmol-k = bar-m 3 /kmol-K = L-atm/kmol-K = Btu/lbmol-R = ft-lbf/lbmol-R = psia-ft 3 /lbmol-R
Thermodynamic Properties Property Table w Property Table -- from direct measurement w Equation of State w Equation of State -- any equations that relates P,v, and T of a substance
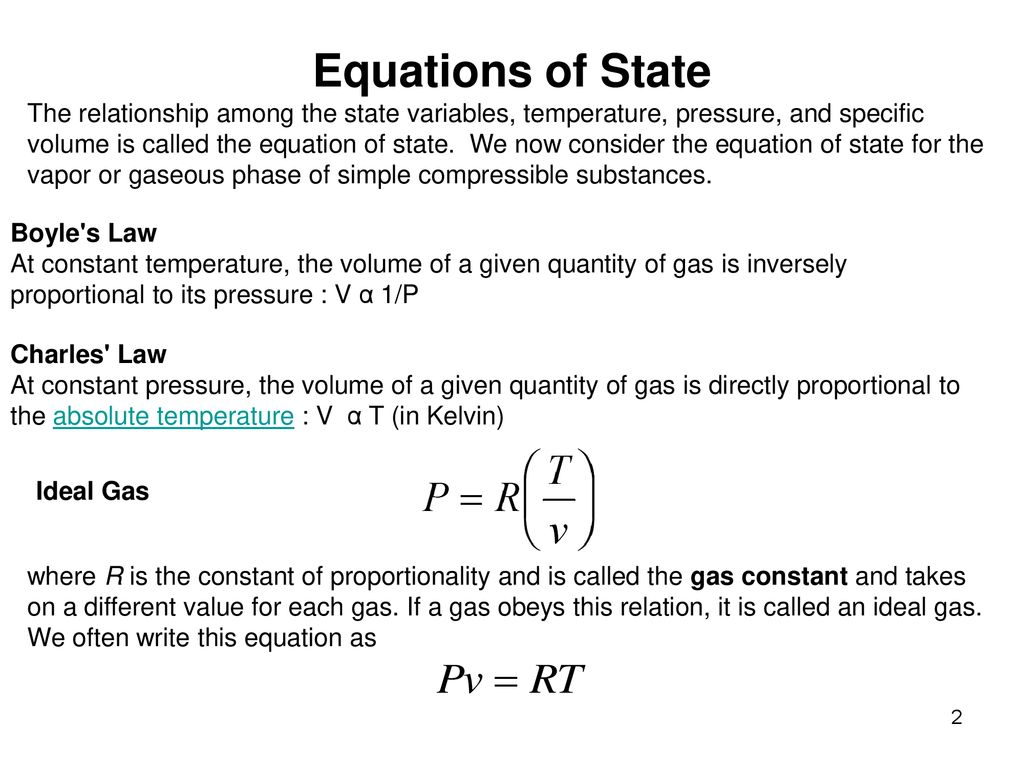
The simplest and best-known equation of state is the ideal-gas equation of state, given as where R is the gas constant. Caution should be exercised in using this relation since an ideal gas is a fictitious substance. Real gases exhibit ideal-gas behavior at relatively low pressures and high temperatures..
Example Determine the particular gas constant for air and hydrogen.
Ideal Gas Law is a simple Equation of State
Percent error for applying ideal gas equation of state to steam
Question … Under what conditions is it appropriate to apply the ideal gas equation of state
w Air, nitrogen, oxygen, hydrogen, helium, argon, neon, carbon dioxide, …. ( < 1% error)..
Compressibility Factor w The deviation from ideal-gas behavior can be properly accounted for by using the compressibility factor Z, defined as Z represents the volume ratio or compressibility.
Ideal Gas Z=1 Real Gases Z > 1 or Z < 1
w Z is known as the compressibility factor. w Real gases, Z 1..
w It accounts mainly for two things Molecular structureMolecular structure Intermolecular attractive forcesIntermolecular attractive forces.
Z = Z(P R,T R ) for all gases.
P cr and T cr are critical properties..
Compressibility factor for ten substances (applicable for all gases Table A-3)
Table A-7 Mol (kg-Mol) R (J/kg.K) Tcrit (K) Pcrit (MPa) Ar28,97287,0(---) O2O2 32,00259,8154,85,08 H2H2 2, ,233,31,30 H2OH2O18,016461,5647,122,09 CO 2 44,01188,9304,27,39.
w However, if you are given P and v and asked to find T (or T and v and asked to find P), trouble lies ahead. w Use pseudo-reduced specific volume..
Pseudo-Reduced Specific Volume w When either P or T is unknown, Z can be determined from the compressibility chart with the help of the pseudo- reduced specific volume, defined as not v cr !
Ideal-Gas Approximation w The compressibility chart shows the conditions for which Z = 1 and the gas behaves as an ideal gas: w (a) P R 1
Other Thermodynamic Properties: Isobaric (c. pressure) Coefficient v T P
Other Thermodynamic Properties: Isothermal (c. temp) Coefficient v P T
Hence infinitesimal differences in volume are expressed as infinitesimal differences in P and T, using and coefficients If and are constant, we can integrate for v:.
Other Thermodynamic Properties: Internal Energy, Enthalpy and Entropy
Other Thermodynamic Properties: Specific Heat at Const. Volume u T v
Other Thermodynamic Properties: Specific Heat at Const. Pressure h T P
Other Thermodynamic Properties: Ratio of Specific Heat
Other Thermodynamic Properties: Temperature s u v T 1
Ideal Gases: u = u(T) Therefore, 0
We can start with du and integrate to get the change in u: Note that C v does change with temperature and cannot be automatically pulled from the integral.
w Therefore, h = u + RT => since u is only a function of T, R is a constant, then h is also only a function of T w so h = h(T).
Similarly, for a change in enthalpy for ideal gases:
w For ideal gases, C v and C p are written in terms of ordinary differentials as.
For an ideal gas, w h = u + Pv = u + RT C p = C v + R
Ratio of specific heats is given the symbol,
Other relations with the ratio of specific heats which can be easily developed:
For monatomic gases, Argon, Helium, and Neon
w Next figure shows the temperature behavior …. specific heats go up with temperature..
Specific Heats for Some Gases w C p = C p (T) a function of temperature
Three Ways to Calculate Δu and Δh w Δu = u 2 - u 1 (table) w Δu = w Δu = C v,av ΔT w Δh = h 2 - h 1 (table) w Δh = w Δh = C p,av ΔT
Isothermal Process w Ideal gas: PV = mRT
For ideal gas, PV = mRT We substitute into the integral Collecting terms and integrating yields:
Polytropic Process w PV n = C
How P,v and T behavior when Q = 0. w To develop an expression to the adiabatic process is necessary employ: 1. Reversible work mode: dW = PdV 2. Adiabatic hypothesis: dQ =0 3. Ideal Gas Law: Pv=RT 4. Specific Heat Relationships 5. First Law Thermodynamics: dQ-dW=dU.
Ideal Gas Adiabatic Process and Reversible Work (cont) First Law: Using P = MRT/V Integrating from (1) to (2)
Ideal Gas Adiabatic Process and Reversible Work (cont) Using the gas law : Pv=RT, other relationship amid T, V and P are developed accordingly:
i and f represent the initial and final states.
For most of the gases, 1.4 w The adiabatic lines are always at the righ of the isothermal lines. w The former is Pv = constant (the exponent is unity) P v Q = 0 T=const. i f f.
Polytropic Process A frequently encountered process for gases is the polytropic process: PV n = c = constant Since this expression relates P & V, we can calculate the work for this path.
Polytropic Process w Constant pressure 0 w Constant volume w Isothermal & ideal gas 1 w Adiabatic & ideal gas k=C p /C v Process Exponent n
Boundary work for a gas which obeys the polytropic equation
We can simplify it further The constant c = P 1 V 1 n = P 2 V 2 n

Chapter Three_ Part Two - ppt download

Thermodynamic Properties Property Table w Property Table -- from

Chapter Three_ Part Two - ppt download

Chapter Three_ Part Two - ppt download

Chapter Three_ Part Two - ppt download

1 Equations of State The relationship among the state variables

1 Equations of State The relationship among the state variables
Thermodynamic Properties Property Table w Property Table -- from

1 Equations of State The relationship among the state variables
Chapter Three_ Part Two - ppt download